
May 7, 2025
| Today’s news and insights for biopharma leaders
A vocal opponent of his predecessor Peter Marks, Vinay Prasad will now lead the office tasked with reviewing some genetic medicines, adding more uncertainty to an already struggling field of research.
|
Already facing headwinds caused by a patient’s death and treatment delays, the biotech now may have to contend with a less friendly FDA.
|
Prasad has gained a reputation for questioning U.S. health policy and accelerated approvals in oncology, and has also opposed some vaccine mandates and the use of COVID shots in children.
|
Drugmakers and, largely, the FDA argue that the medicines now being reviewed are different than the chemotherapies and blunter interventions of the past. Explore the latest in oncology research in
|
The maker of Ozempic and Wegovy expects lower sales and profit growth this year despite recent victories against compounders.
|
The advisory committee meeting is an important step in the process of readying boosters for the fall and winter season, but will take place amid newly imposed scrutiny of COVID shots.
|
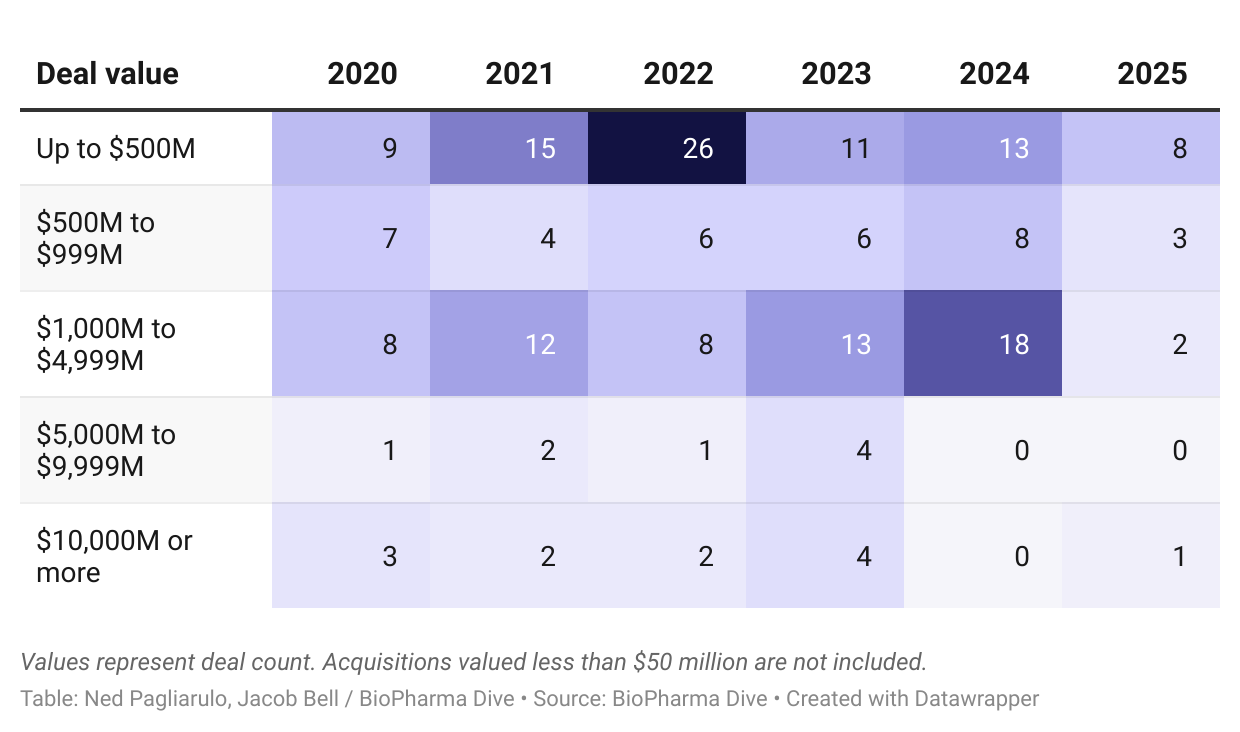
Experts say market turmoil has made it harder for buyers and sellers to agree on price, which can be an especially imposing obstacle to large acquisitions.
|

|
Stronger connections lead to better outcomes. In this infographic, learn how to use key moments in the product lifecycle, from drug discovery to commercialization, to help transform patient care.
|
|
From Our Library
Trendline
Supported by Phil Inc
|
Trendline
Supported by EVERSANA
|
Trendline
Supported by Catalent
| |